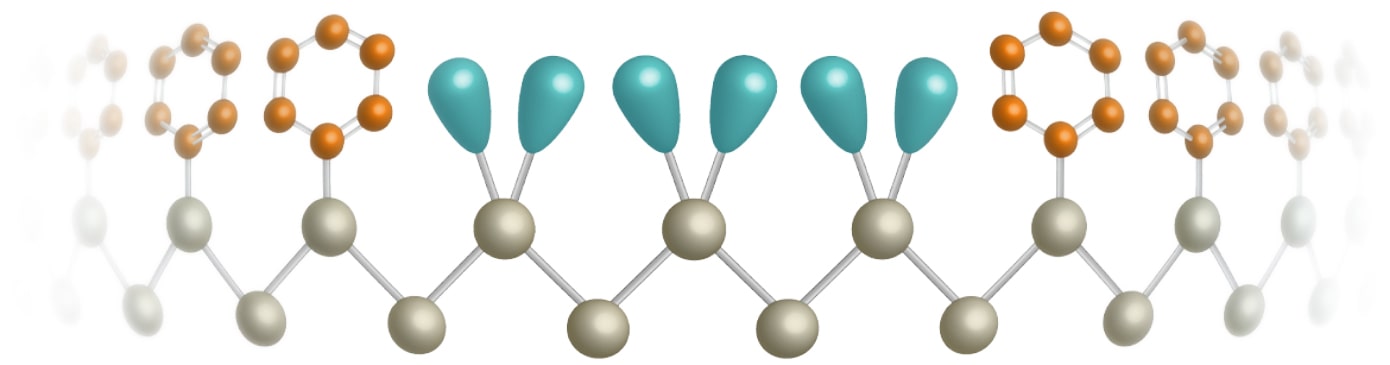
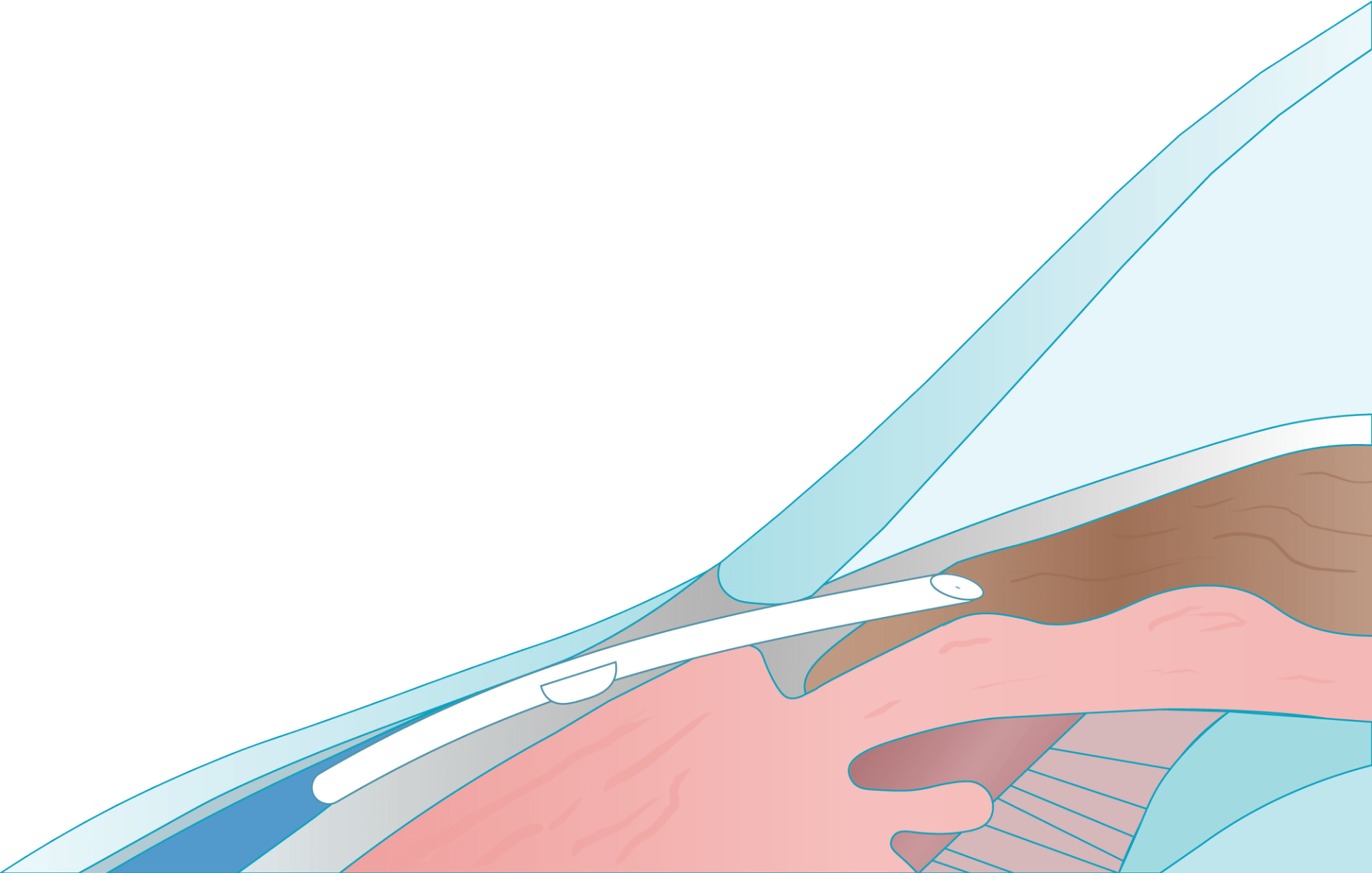
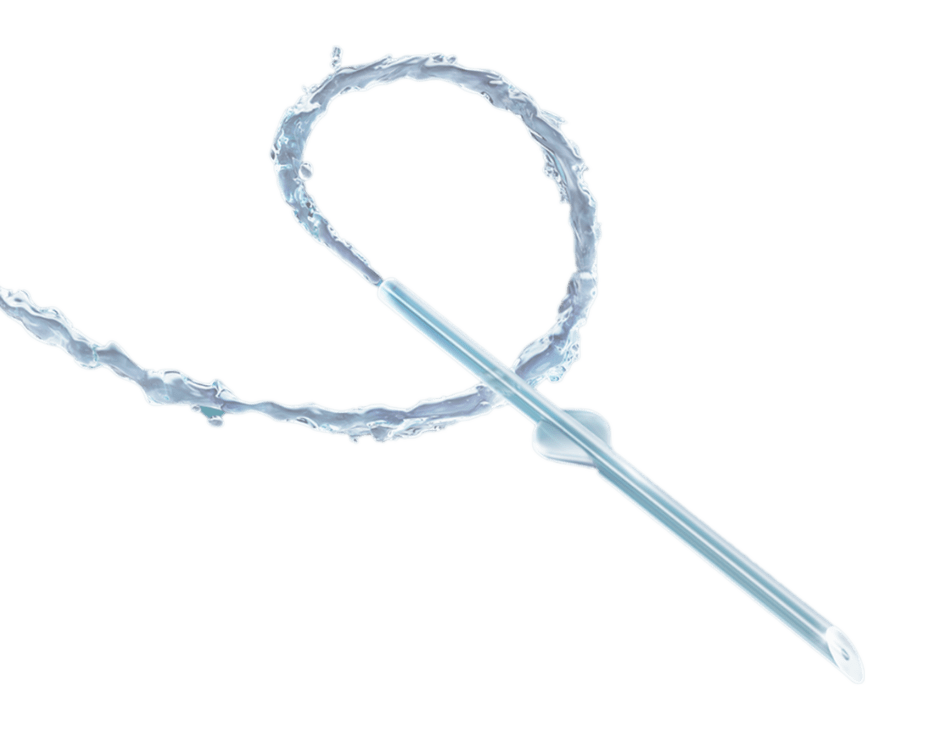
Contraindications
The implantation of the PRESERFLO™ MicroShunt is contraindicated if one or more of the following conditions exist: BACTERIAL CONJUNCTIVITIS; BACTERIAL CORNEAL ULCERS; ENDOPHTHALMITIS; ORBITAL CELLULITIS; BACTEREMIA OR SEPTICEMIA; ACTIVE SCLERITIS; UVEITIS; SEVERE DRY EYE; SEVERE BLEPHARITIS; PRE-EXISTING OCULAR OR SYSTEMIC PATHOLOGY THAT, IN THE OPINION OF THE SURGEON, IS LIKELY TO CAUSE POSTOPERATIVE COMPLICATIONS (E.G. SEVERE MYOPIA AND THIN CONJUNCTIVA) FOLLOWING IMPLANTATION OF THE DEVICE; PATIENTS DIAGNOSED WITH ANGLE CLOSURE GLAUCOMA.